 When Endocrine Society past-president Richard J. Santen, MD, achieved “emeritus” status at the University of Virginia in Charlottesville, he was far from being done with practicing medicine. That’s when he decided to continue seeing patients throughout southwest Virginia from the comfort of his home and with help from telehealth technology and the Federally Funded Community...
When Endocrine Society past-president Richard J. Santen, MD, achieved “emeritus” status at the University of Virginia in Charlottesville, he was far from being done with practicing medicine. That’s when he decided to continue seeing patients throughout southwest Virginia from the comfort of his home and with help from telehealth technology and the Federally Funded Community...Out of Practice: Why Retirement Doesn’t Always Mean You Stop Seeing Patients
 When Endocrine Society past-president Richard J. Santen, MD, achieved “emeritus” status at the University of Virginia in Charlottesville, he was far from being done with practicing medicine. That’s when he decided to continue seeing patients throughout southwest Virginia from the comfort of his home and with help from telehealth technology and the Federally Funded Community...
When Endocrine Society past-president Richard J. Santen, MD, achieved “emeritus” status at the University of Virginia in Charlottesville, he was far from being done with practicing medicine. That’s when he decided to continue seeing patients throughout southwest Virginia from the comfort of his home and with help from telehealth technology and the Federally Funded Community...
 This month, The Journal of Clinical Endocrinology & Metabolism is publishing “Appropriate Use of Telehealth Visits in Endocrinology: Perspective Statement of the Endocrine Society.” Since this new modality is here to stay, Endocrine News talks to the authors about which patients are better candidates for telehealth than others, why endocrinology seems tailor-made for this new...
This month, The Journal of Clinical Endocrinology & Metabolism is publishing “Appropriate Use of Telehealth Visits in Endocrinology: Perspective Statement of the Endocrine Society.” Since this new modality is here to stay, Endocrine News talks to the authors about which patients are better candidates for telehealth than others, why endocrinology seems tailor-made for this new... Endocrine research remains at the forefront of medical breakthroughs, which has led to cutting-edge treatment options, therapies, and products. Here is part 2 of Endocrine News’s closer look at some of the endocrine science innovations announced throughout 2021. Endocrine researchers and clinicians continued to remain at the forefront of medical research, the result of which...
Endocrine research remains at the forefront of medical breakthroughs, which has led to cutting-edge treatment options, therapies, and products. Here is part 2 of Endocrine News’s closer look at some of the endocrine science innovations announced throughout 2021. Endocrine researchers and clinicians continued to remain at the forefront of medical research, the result of which...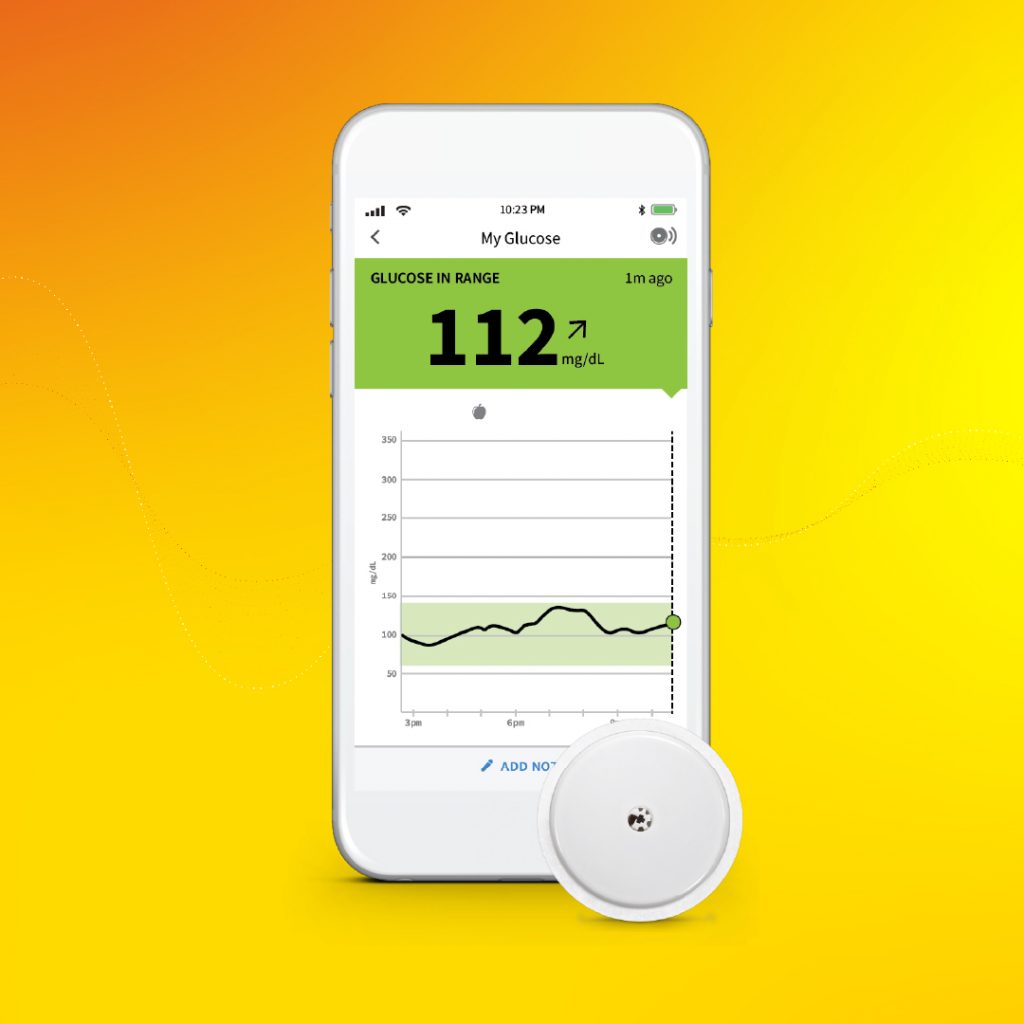 The U.S. Food and Drug Administration this week cleared Abbott’s FreeStyle® Libre 2 iOS application for use with compatible iPhones, to pair with its FreeStyle Libre 2 integrated continuous glucose monitoring (iCGM) system. Approved for adults and children (4 and older) with diabetes, the new app enables users to get glucose readings directly on their...
The U.S. Food and Drug Administration this week cleared Abbott’s FreeStyle® Libre 2 iOS application for use with compatible iPhones, to pair with its FreeStyle Libre 2 integrated continuous glucose monitoring (iCGM) system. Approved for adults and children (4 and older) with diabetes, the new app enables users to get glucose readings directly on their...