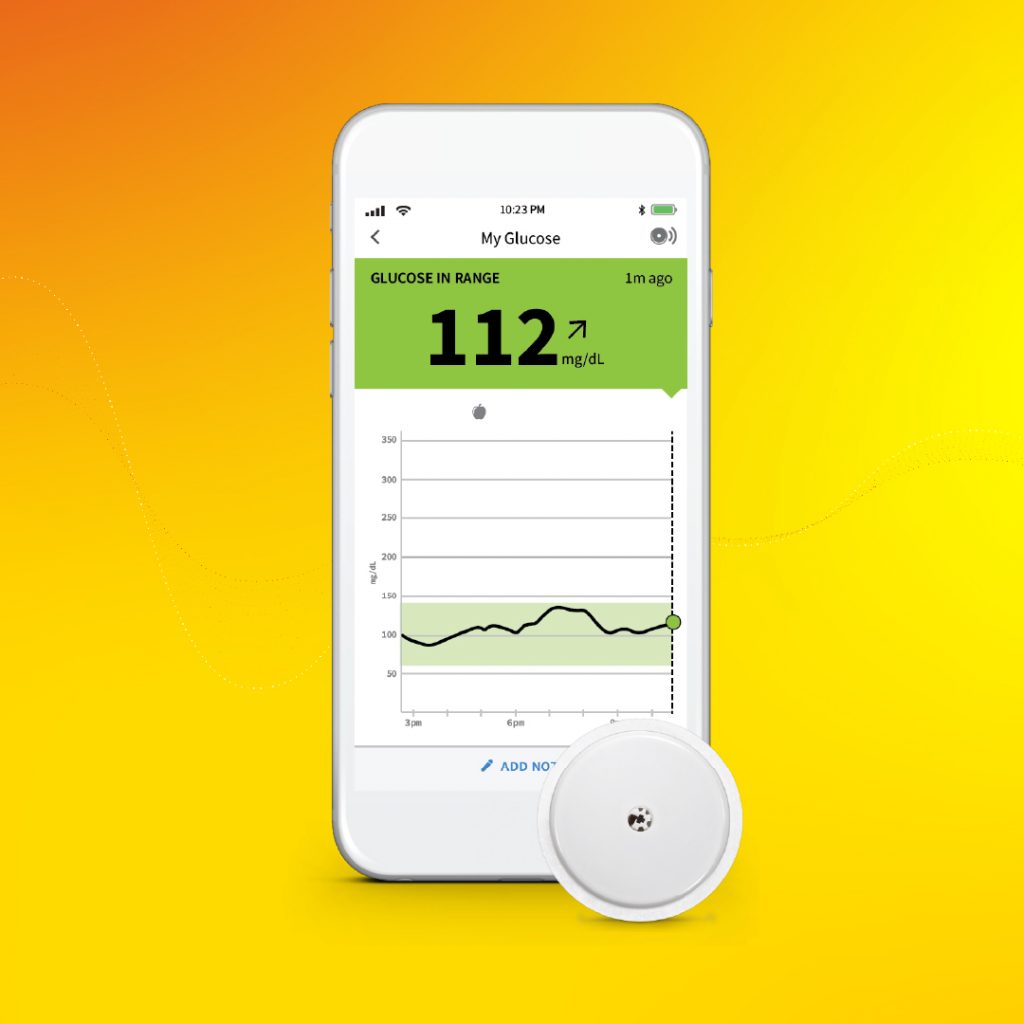
The U.S. Food and Drug Administration this week cleared Abbott’s FreeStyle® Libre 2 iOS application for use with compatible iPhones, to pair with its FreeStyle Libre 2 integrated continuous glucose monitoring (iCGM) system.
Approved for adults and children (4 and older) with diabetes, the new app enables users to get glucose readings directly on their iPhones without the use of a reader.

“The pandemic taught us the importance of connected devices in managing chronic conditions remotely, and we continue to see the benefit from the latest advances in digital health,” says Endocrine Society member Kurt Midyett, MD, pediatric endocrinologist and medical director at Midwest Pediatric Specialists. “Continuous glucose monitoring is one of the most significant health tech innovations in the last decade, and I see the life-changing benefits first-hand from my patients every day. As a doctor, it’s invaluable to receive my patients’ glucose data remotely via systems like LibreView – enabling more meaningful conversations with my patients, and ultimately, improving treatment decisions and their long-term health.”

