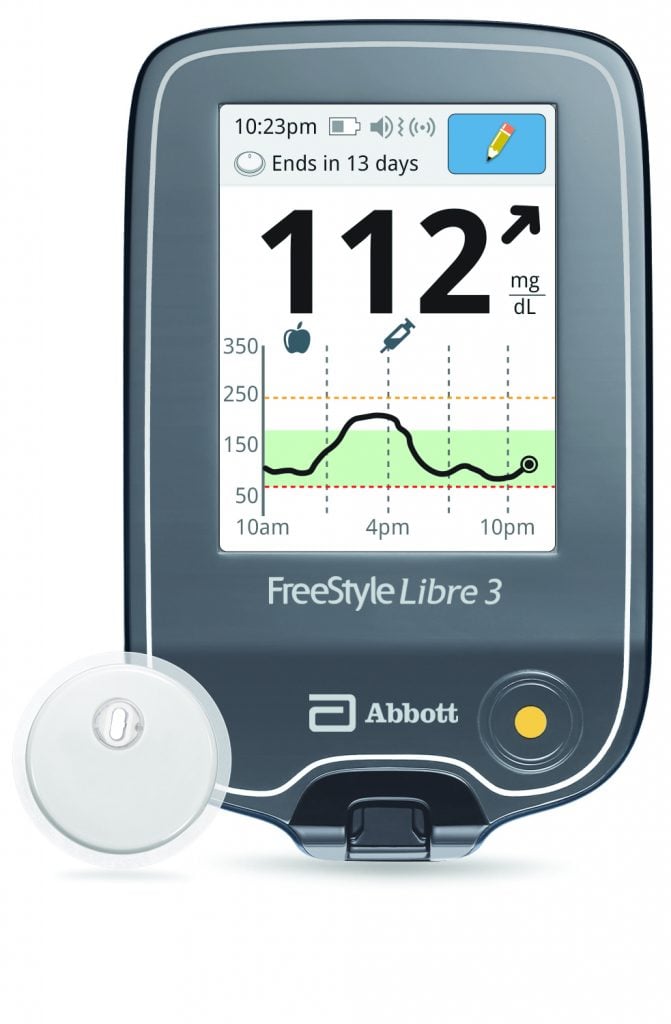
 Abbott announced that the U.S. Food and Drug Administration (FDA) has cleared a reader for its FreeStyle Libre® 3 integrated continuous glucose monitoring (iCGM) system, which features the world’s smallest, thinnest, and most discreet glucose sensor. With the FDA’s clearance of a standalone reader, Abbott is working to get the FreeStyle Libre 3 system added to Medicare’s...
Abbott announced that the U.S. Food and Drug Administration (FDA) has cleared a reader for its FreeStyle Libre® 3 integrated continuous glucose monitoring (iCGM) system, which features the world’s smallest, thinnest, and most discreet glucose sensor. With the FDA’s clearance of a standalone reader, Abbott is working to get the FreeStyle Libre 3 system added to Medicare’s...Glytec Announces Next Evolution of GlucoMetrics as Hospitals Prepare for New CMS Measures
Eli Lilly Cuts Insulin Prices by 70% and Caps Patient Insulin Out-of-Pocket Costs at $35 Per Month
Out of Practice: Why Retirement Doesn’t Always Mean You Stop Seeing Patients
 When Endocrine Society past-president Richard J. Santen, MD, achieved “emeritus” status at the University of Virginia in Charlottesville, he was far from being done with practicing medicine. That’s when he decided to continue seeing patients throughout southwest Virginia from the comfort of his home and with help from telehealth technology and the Federally Funded Community...
When Endocrine Society past-president Richard J. Santen, MD, achieved “emeritus” status at the University of Virginia in Charlottesville, he was far from being done with practicing medicine. That’s when he decided to continue seeing patients throughout southwest Virginia from the comfort of his home and with help from telehealth technology and the Federally Funded Community...The endocrine system is a miracle of evolution — a highly complex machine that carries out multiple functions within the body with precision and efficiency. It’s no wonder that as our understanding of the endocrine system advances, the technology we use to study and care for it also becomes more advanced as it approaches the endocrine system in terms of sophistication. Whether that endocrinology technology takes the form of new, advanced medicines, sophisticated imaging and diagnostic technology, or innovative therapies that make life easier for patients, endocrine science continues to push the boundaries and enter new territory. For endocrinologists, staying informed about all of the latest advances in technology and effective treatment options mean better overall care for their patients.
This section compiles the latest news regarding technological advances in endocrinology, featuring articles written for and by the leading voices in endocrine science today. Here, you’ll find articles relating to all the most recent happenings in technology, techniques and general information of interest to endocrinologists. Keeping up with the latest advancements in the field will help you know where medicine has been, where it will advance next, and how it will look in the future. Endocrine News brings you everything you need to know about the future of endocrinology so you’ll know exactly when science catches up to your patients’ needs. The field of endocrinology continues to advance, and Endocrine News is your source for advancing your own knowledge of everything that’s going on for today and tomorrow.

