Dosage Flexibility of Liquid Hypothyroidism Treatment Introduced for Patients and Clinicians
Americans diagnosed with hypothyroidism and their clinical providers now have access to greater dosage flexibility within levothyroxine therapy, with three new dosage strengths of levothyroxine sodium oral solution now available to treat hypothyroidism. IBSA Pharma markets the drug as Tirosint®-SOL. The unique new dosing options – 37.5, 44 and 62.5 micrograms – are a first...

 The discovery of a new mutation could eventually hold the key to treating patients with adrenal carcinoma. Endocrine News talks with Emilia Pinto, PhD, a St. Jude’s Children’s Research Hospital scientist, who hopes that this new finding will raise awareness around the world in families who might be at a greater risk for this rare...
The discovery of a new mutation could eventually hold the key to treating patients with adrenal carcinoma. Endocrine News talks with Emilia Pinto, PhD, a St. Jude’s Children’s Research Hospital scientist, who hopes that this new finding will raise awareness around the world in families who might be at a greater risk for this rare... Patients with classic congenital adrenal hyperplasia (CAH) due to 21-hydroxylase deficiency (21OHD) may benefit from the drug tildacerfont, according to the results from two Phase 2 clinical studies recently published in The Journal of Clinical Endocrinology & Metabolism. Researchers led by Chris N. Barnes, PhD, of Spruce Biosciences (who funded the trial), point out that...
Patients with classic congenital adrenal hyperplasia (CAH) due to 21-hydroxylase deficiency (21OHD) may benefit from the drug tildacerfont, according to the results from two Phase 2 clinical studies recently published in The Journal of Clinical Endocrinology & Metabolism. Researchers led by Chris N. Barnes, PhD, of Spruce Biosciences (who funded the trial), point out that...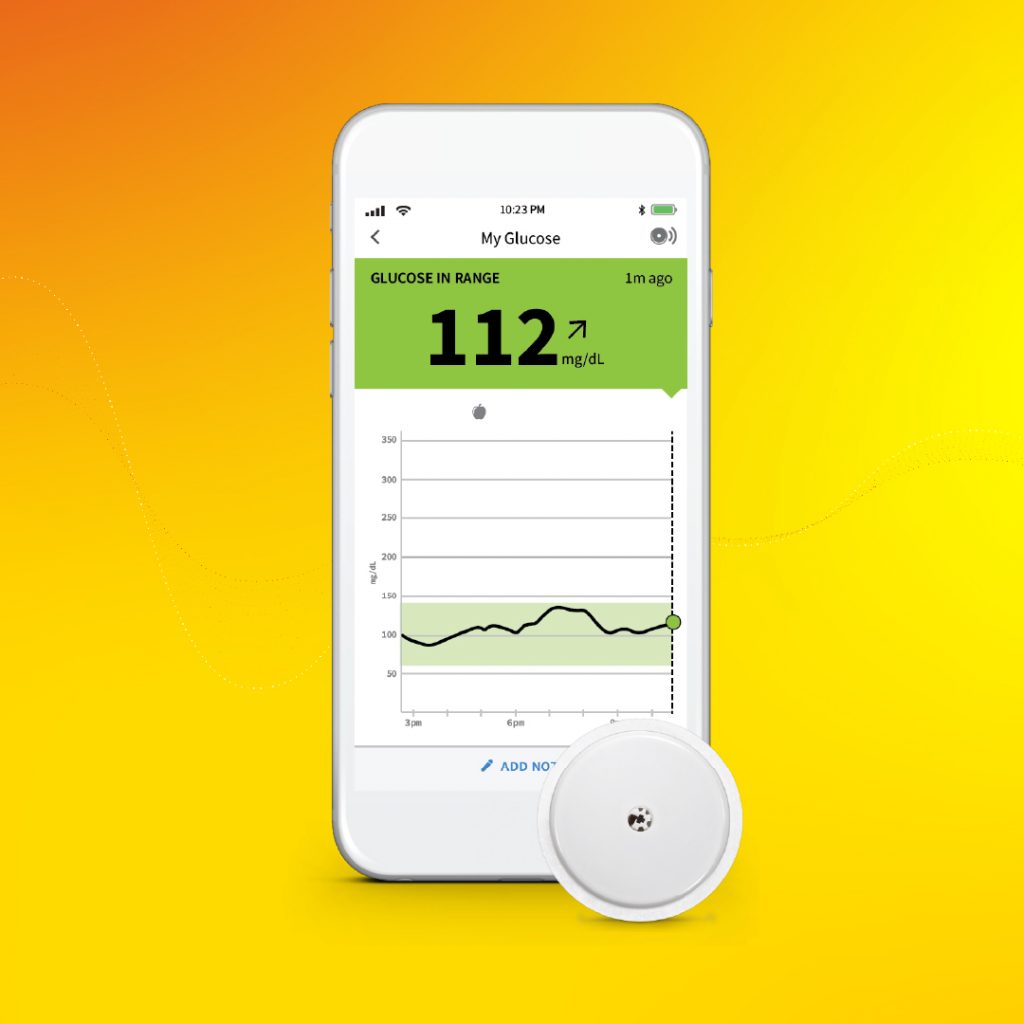 The U.S. Food and Drug Administration this week cleared Abbott’s FreeStyle® Libre 2 iOS application for use with compatible iPhones, to pair with its FreeStyle Libre 2 integrated continuous glucose monitoring (iCGM) system. Approved for adults and children (4 and older) with diabetes, the new app enables users to get glucose readings directly on their...
The U.S. Food and Drug Administration this week cleared Abbott’s FreeStyle® Libre 2 iOS application for use with compatible iPhones, to pair with its FreeStyle Libre 2 integrated continuous glucose monitoring (iCGM) system. Approved for adults and children (4 and older) with diabetes, the new app enables users to get glucose readings directly on their... Seventeen voicemails, 14 boxes of Endocrine News, and a Costa Coffee tumbler bought in London in 2019 that was in dire need of a good scrubbing. That’s what awaited me when I returned to my office at the Endocrine Society headquarters in Washington D.C. for the first since March 13, 2020. Honestly, it was good...
Seventeen voicemails, 14 boxes of Endocrine News, and a Costa Coffee tumbler bought in London in 2019 that was in dire need of a good scrubbing. That’s what awaited me when I returned to my office at the Endocrine Society headquarters in Washington D.C. for the first since March 13, 2020. Honestly, it was good...