Cutting-edge advancements in endocrinology continue to move diabetes treatment in the right direction faster than ever before.
The treatment of diabetes is continually evolving. More than a century ago, a diabetes diagnosis was a death sentence, and although there is not yet a cure, endless updates in diabetes care help ease the burden of both the patient and the provider. Although hurdles remain, ranging from expensive medications and devices to a lack of understanding of what’s expected for optimal care, medical advancements continue to make diabetes more manageable than ever. Here, we look at some of the latest products endocrinologists and their patients rely on to treat diabetes.
Tempo

The Tempo™ Personalized Diabetes Management Platform consists of three key components – the Tempo Smart Button®; a compatible app, TempoSmart™; and a prefilled insulin pen, Tempo Pen® – which work together to deliver personalized guidance for adults with diabetes. The Tempo Smart Button™ attaches to the Tempo Pen™ and pairs with the TempoSmart™ App for insulin dose-related data to be transferred to a smartphone. The app combines this data with readings from the Tempo Blood Glucose Meter (BGM) and/or Dexcom Continuous Glucose Monitoring (CGM) System and provides personalized progress reports to assist with the self-management of diabetes.
Diabeloop

The DBLG1 self-learning algorithm developed by Diabeloop automates and personalizes the treatment of type 1 diabetes. DBLG1 is hosted on a dedicated handset, the user interface, and is connected to a continuous glucose monitor (CGM) and insulin pump. Every five minutes, a glucose measurement is transmitted via Bluetooth® technology to the handset, which the DBLG1 artificial intelligence analyzes in real time, while considering the patient’s physiology, history, and data entries (meals or exercise) to determine the correct dose of insulin to administer.
iLet Bionic Pancreas

In May 2023, Beta Bionics, Inc. announced FDA 510(k) clearance and the commercial launch of the iLet Bionic Pancreas. The iLet is the first and only automated insulin-delivery system that does not require carb counting and fully automates 100% of all user insulin doses, resulting in no calculations, less input, and less burden for the user. Paired with the Dexcom G6 Continuous Glucose Monitoring System, the iLet was designed to alleviate the work of diabetes management in everyday life and nearly eliminate the expertise that has been required to set up and manage a traditional insulin pump. With the iLet, healthcare providers can spend less time with technology and more time with their patient. The only input that is required to get started is the user’s weight — the iLet does the rest.
Mallya
Another product that recently secured clearance from the FDA is BIOCORP’s Mallya smart medical device. Mallya is a smart sensor that has been designed to be attached directly to insulin pen injectors, converting them into connected devices. It automatically records important treatment information, including selected insulin units as well as the date and time of injection, and transmits the data to a dedicated digital app. Mallya is claimed to be the first device capable of automatically connecting different insulin and GLP-1 drugs to be approved in the U.S.

Eversense E3
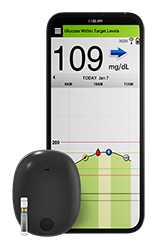
The only implantable sensor for long-term wear, the Eversense® E3 offers continuous glucose readings for up to six months. Its removable and rechargeable smart transmitter delivers on-body vibration alerts when there are high or low glucose values, providing patients with diabetes discretion and peace of mind. The system also features a convenient app for real-time diabetes monitoring and management. The Smart Transmitter wirelessly powers the sensor. This activates the sensor, which flashes an LED light source. A fluorescent polymer coating on the outside of the sensor responds to the LED light. Glucose from the body reversibly binds to the coating and the amount of light emitted by the coating rises and falls with the body’s glucose concentration. This emitted light is measured by photodetectors inside the sensor. Special circuitry digitizes the measurements and sends the data to the smart transmitter, which calculates the glucose value, direction, and rate of change and sends the information to the mobile app. While the current model lasts six months, current trials are now underway to extend use of the sensors to a full year.
DISCLAIMER: INCLUSION IN THIS COLUMN DOES NOT SUGGEST AN ENDORSEMENT BY ENDOCRINE NEWS OR THE ENDOCRINE SOCIETY.

