An Endocrine News roundup of the week’s pharmaceutical news, breakthroughs, and general information. *
Rezolute Provides Insights from its Phase 3 sunRIZE Study in Congenital Hyperinsulinism and Shares Findings from its Expanded Access Program in Tumor Hyperinsulinism
On January 7, Rezolute, Inc., a late-stage rare disease company focused on treating hypoglycemia caused by all forms of hyperinsulinism (HI), shared observations from the Phase 3 sunRIZE study in patients with congenital HI and provided details on the treatment of tumor HI patients with ersodetug under the Company’s EAP.
While sunRIZE did not meet its primary (hypoglycemia events) or key secondary (time in hypoglycemia) endpoints, the Company believes that the totality of the data further supports previous clinical evidence that ersodetug is active against hypoglycemia in patients. Specifically, there was evidence of pharmacologic activity as target therapeutic drug concentrations were achieved in both treatment groups (5 mg/kg and 10 mg/kg) with highly sensitive biomarker responses (increases in circulating insulin) in the active treatment groups that are indicative of reduced insulin activity at its receptor. Notably, these responses were consistent with those of the Company’s Phase 2 RIZE study (see Figure 1).
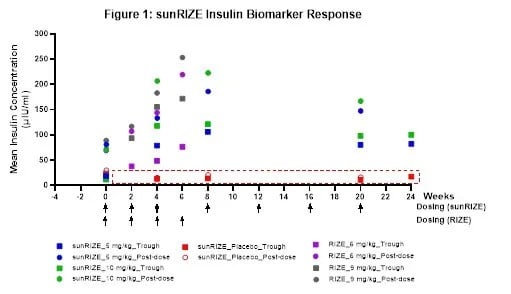
The study also demonstrated reductions from baseline in events and time in hypoglycemia in both treatment groups, but not enough to be statistically significant compared to the pronounced study effect in the placebo arm. While in the early stages of evaluating study data and understanding the results, learnings in the field of glycemic control and initial observations from sunRIZE inform the Company’s belief that the pharmacologic response can translate to clinical efficacy. The magnitude of the placebo response observed for hypoglycemia events reveals a significant challenge in studying glucose in an ambulatory setting, where factors such as intensive monitoring where caregivers receive alerts regarding hypoglycemic events and frequent clinical interactions can independently influence outcomes.
The Company believes that the extent of reduction from baseline in hypoglycemia events and time in hypoglycemia relative to placebo may have been impacted by the prolonged treatment duration of six months and the fact that glucose monitoring is necessary for safe patient management while also serving as the key endpoint in the study. This sentiment has been shared with the Company by investigator physicians as well as study participants. The Company is currently exploring how to characterize the overall study dynamic including evaluating patient-reported quality of life outcomes.
In light of these limitations, assessing the potential benefit in the ongoing open-label extension (OLE) portion of the study will be important. All 59 participants who completed the study elected to continue to receive ersodetug in the OLE. To date, 57 participants remain in the OLE, with an exposure duration ranging from ~6 weeks for the most recently entered patients, to ~18 months. The Company believes that a potential indicator of ersodetug’s underlying efficacy is that several children in the OLE have been able to stop taking all other therapies and are now receiving ersodetug as monotherapy.
The Company looks forward to interacting with FDA in Q1 2026 under its Breakthrough Therapy Designation to further characterize these and other clinical outcomes to inform a review of the full sunRIZE dataset with the intent of exploring options for this indication.
Tumor HI
Over the past two years, Rezolute has collaborated with investigators across the United States and in Europe to provide ersodetug to more than a dozen patients with severe and refractory hypoglycemia due to tumor HI, including malignant pancreatic neuroendocrine tumors (insulinomas) and non-islet cell tumors. The Company has previously reported that the therapy was generally well-tolerated, and that patients experienced substantial improvement in hypoglycemia, which led to a reduction in the rate of glucose infusion in the hospital (GIR) or the complete discontinuation of infusion and discharge from the hospital.
Presented in a table filed today on Form 8-K with the U.S. Securities and Exchange Commission are cumulative data from the initial 9 participants in the EAP, including patient characteristics, ersodetug dosing, and observed outcomes. This same data cohort was provided to FDA last year in support of the Company’s request for Breakthrough Therapy Designation and subsequently informed the discussion with FDA that led to revision of the Phase 3 upLIFT study in tumor HI to a single arm, open-label study. In summary, 75% of the patients receiving IV dextrose/total parental nutrition (TPN) in the EAP achieved a complete discontinuation of IV dextrose/TPN.
This outcome is highly relevant to the ongoing upLIFT study and provides additional evidence of the activity and potential efficacy of ersodetug across various forms of HI. Notably, the GIR assessment in the EAP is the primary endpoint in upLIFT, which measures the number of participants (out of ~16) who achieve at least a 50% reduction in GIR, an objective endpoint in a highly controlled hospital setting. For statistical significance, 9 of 16 open-label participants need to achieve this threshold. Topline results are anticipated in the second half of 2026.
About sunRIZE
The Phase 3 sunRIZE study (RZ358-301) was a multi-center, randomized, double-blind, placebo-controlled, parallel arm study designed to evaluate the efficacy and safety of ersodetug in patients with congenital hyperinsulinism (HI), ages 3 months to 45 years old, who were experiencing continued hypoglycemia on currently available standard of care (SOC). Eligible participants were randomized to one of three treatment arms to receive either ersodetug (5 or 10 mg/kg) or matched placebo-control as add on to existing SOC. Study drug was administered every other week during an initial loading phase, and then every 4 weeks during the 6-month controlled pivotal treatment period. Following the pivotal treatment phase of the study, participants could roll-over into an optional open-label extension phase to continue to receive ersodetug.
The study enrolled 63 participants in more than a dozen countries around the world, inclusive of U.S. patients. The primary and key secondary efficacy endpoints in the study were the change from baseline in the average number of hypoglycemia events per week and the average percent time in hypoglycemia, respectively, over six months of treatment.
Amylyx Pharmaceuticals Announces Nomination of AMX0318 as a Novel, Long-Acting GLP-1 Receptor Antagonist Development Candidate, Identified in Collaboration with Gubra A/S
On January 8, Amylyx Pharmaceuticals, Inc., announced the selection of AMX0318, a long-acting glucagon-like peptide-1 (GLP-1) receptor antagonist, as a development candidate for post-bariatric hypoglycemia (PBH) and other rare diseases. AMX0318 was identified through a research collaboration with Gubra A/S (“Gubra”), a company specializing in peptide-based drug discovery and preclinical contract research services.
“We are very pleased to nominate AMX0318 as a development candidate and were highly impressed by Gubra’s proprietary and innovative process, which identified a peptide that surpassed our research target profile for a long-acting GLP-1 receptor antagonist. AMX0318 has thus far shown robust preclinical and chemical properties, including a pharmacokinetic profile that may support long-acting administration,” says Endocrine Society member Camille L. Bedrosian, MD, Chief Medical Officer at Amylyx. “We are excited about the opportunity to develop additional therapeutic possibilities for people who may benefit from inhibiting GLP-1 receptor activity, including people with PBH and other rare diseases. The GLP-1 receptor is a well-characterized biological target and one of the key regulators of glucose-insulin homeostasis.”
Bedrosian continued, “We have strong conviction that inhibiting GLP-1 receptor activity represents an important therapeutic approach given the statistically significant data that avexitide, our investigational, first-in-class GLP-1 receptor antagonist, has generated to date. We continue to expect completion of recruitment in our pivotal Phase 3 LUCIDITY trial of avexitide in Q1 2026, with topline data expected in Q3 2026.”
“We are excited about the opportunity to develop additional therapeutic possibilities for people who may benefit from inhibiting GLP-1 receptor activity, including people with PBH and other rare diseases. The GLP-1 receptor is a well-characterized biological target and one of the key regulators of glucose-insulin homeostasis.” – Endocrine Society member Camille L. Bedrosian, MD, Chief Medical Officer at Amylyx
AMX0318 has completed extensive preclinical evaluation, including stability, solubility, potency, in vivo pharmacokinetics and pharmacodynamics, and in vivo tolerability studies. Amylyx expects the program to advance into investigational new drug (IND)-enabling studies later this year, with an IND targeted for 2027, pending successful completion of IND-enabling studies.
“We are pleased to see AMX0318 advance as a development candidate, a milestone that reflects both the strong collaboration between the Gubra and Amylyx teams and the capabilities of Gubra’s proprietary peptide drug discovery platform,” said Louise S. Dalbøge, Chief Science Officer at Gubra. “Our AI-driven streaMLine platform enables multi-parameter optimization of peptide candidates, and we are excited to see this applied successfully to AMX0318 in close collaboration with Amylyx.”
Under the terms of the research collaboration, Gubra is eligible to receive more than $50 million in success-based development and commercialization milestones plus mid-single digit royalties on worldwide net sales. The selection and handover of the development candidate will provide milestone payments of $4 million to Gubra.
Interim Clinical Results Shows First-in-Human Non-Fibrotic Engraftment and Viable Encapsulated Human Islets in Subjects with Type 1 Diabetes
On January 6, Encellin, a biotechnology company pioneering Encapsulated Cell Replacement Therapy (ENCRT), announced interim clinical results from its ongoing Phase 1 investigational trial (NCT06408311) in study subjects with type 1 diabetes. Analyses of explants from the initial five subjects at prespecified timepoints demonstrated non-fibrotic engraftment with robust vascularization around the ENCRT implant after a 4-month implantation. Importantly, in the initial evaluated explant Encellin observed viable islets, providing the first clinical support that therapeutic cells can persist within the ENCRT implant in humans.
These early findings represent the first reported human evidence suggesting that Encellin’s proprietary ENCRT can potentially be used to mitigate fibrosis, a challenge that has historically limited encapsulated approaches. Moreover, the presence of viable encapsulated human islets suggests that by addressing the fibrotic response, the local microenvironment created by the ENCRT may be capable of supporting living therapeutic cells in the human body, an important step towards the development of cell-based therapies for patients with Type 1 Diabetes.
“These findings represent an exciting milestone for our team and the field,” said Crystal Nyitray, PhD, CEO and Founder of Encellin. “While much work remains, these findings suggest that Encellin’s proprietary ENCRT approach may be capable of supporting cell therapies in humans. If confirmed in larger studies, these observations provide an important foundation for the continued development of ENCRT in Type 1 Diabetes and, over time, other endocrine indications.”
“Outside the body, we can now engineer cells in all sorts of ways. For example, we can engineer them to be little factories producing medicines like hormones and therapeutic peptides in response to outside signals,” said Alex Morgan, Partner at Khosla Ventures. “However, what we can’t currently do is easily keep these foreign cells alive inside the human body. Showing that foreign cells can be kept alive for months within these implanted packets points to a possible future where instead of needing to take medications via an injection or pill, they are just made within us, as needed, from universal cell lines. Demonstration that non-fibrotic, vascularized integration can be achieved and foreign cell viability sustained is a key milestone toward this goal, although there is still a lot of work ahead.”
The Phase 1 trial is designed to evaluate the safety and tolerability of ENCRT devices containing therapeutic cell cargo. Specifically, in this case, subjects were patients with Type 1 Diabetes implanted with allogeneic islets. While analyses are ongoing, these initial observations show early supportive human evidence of non-fibrotic, vascularized integration of ENCRT with viable encapsulated human islets—important foundations for future encapsulated cell-based therapies. Beyond Type 1 Diabetes, Encellin is exploring its ENCRT platform in additional endocrine and metabolic indications, assessing its potential as a modular and scalable solution with the long-term vision of enabling cell-based therapies across multiple diseases.